
Impulse by ICO lance AIforHer, un challenge inédit entre startups et personnel médical qui associe l’intelligence artificielle à l’humain pour accélérer la lutte contre le cancer du sein

Breast cancer treatment is improving all the time, but that’s not enough: we need to diagnose the disease more accurately and target treatments more effectively. With the arrival of new targeted therapies for patients with low or very low expression of the HER2 receptor, there is a clinical imperative to refine and make more reliable the diagnosis of this biomarker, which has become central to therapeutic decisions.
This demand for precision is matched by another upheaval: the rise of digital pathology and artificial intelligence algorithms capable of assisting pathologists in the detailed assessment of HER2. While these technologies are promising, their deployment in routine clinical practice still raises crucial questions: reliability, robustness, interpretability and acceptability to professionals.
It is at this juncture, between therapeutic progress and technological innovation, that a strong need is emerging on the ground: to ensure that the AI tools now available meet clinical requirements and are effectively integrated into medical practices.
To meet this challenge, a number of players are mobilising, drawing on their complementary strengths and their shared commitment to innovation for the benefit of patients:
Together, these partners are launching AI for Her, a call for solutions to identify, test and develop the best AI technologies applied to digital pathology in breast cancer.
The aim is clear : to enable every patient to benefit from the most appropriate therapy, as early as possible.

AI for Her
The pioneers of the greatest scientific discoveries and advances did not set out alone. Positive competition, team spirit and collective ambition in the service of a better tomorrow have been extraordinary drivers of development.
AI for Her aims to be an unprecedented challenge and a collective cooperative movement bringing together the worlds of technology and science to improve the care and treatment of breast cancer patients and help medical staff by bringing ever greater precision, objectivity and reproducibility to their diagnoses.
For a period of 6 to 9 months, anatomopathologists and start-ups developing an artificial intelligence solution capable of scoring HER2 (one of the Human Epidermal growth factor Receptor typologies playing a key role in breast cancer) at low and ultra-low expression levels, will be combining their expertise and focusing their energies on evaluating the performance and use of AI algorithms.
AI for Her is the very first challenge to enable AI algorithms to be evaluated in real life, at the heart of the hospital, thanks to the organisation of an entire cancer centre.
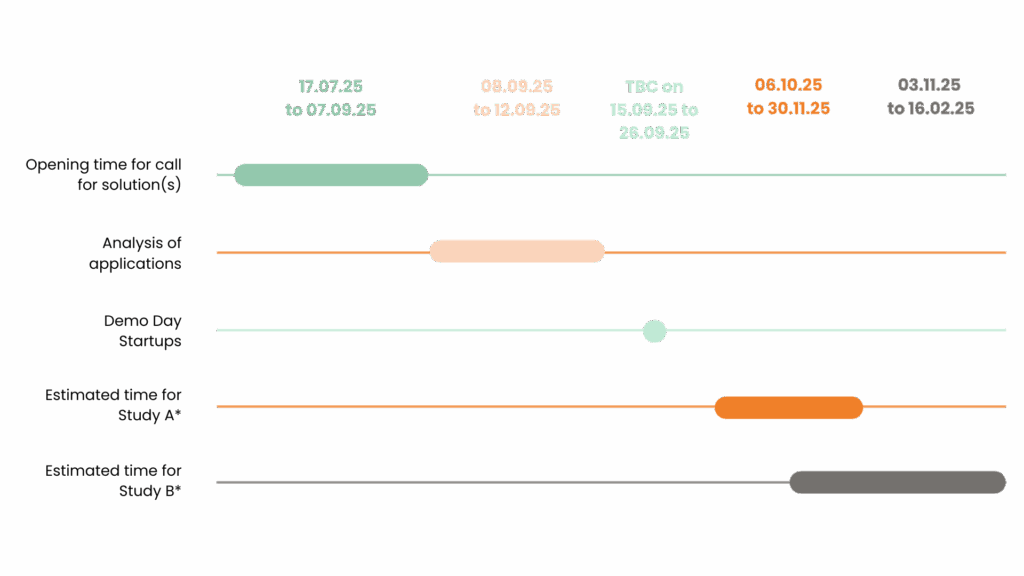
*The launch of studies A and B is subject to contractual agreement with all stakeholders.
For Study A, the timeframe indicated is that of the complete study including statistical analysis of the results. The duration of the scoring of the algorithms is to be determined, but will be spread over a shorter period (a few days).
For study B, the timeframe indicated concerns the three scoring stages (without AI, with AI A, with AI B), each of which will be spread over a few days, with a month’s wash-out planned between each stage to ensure the objectivity of the results. The timeline could extend to the end of February, including the analysis of the final results.

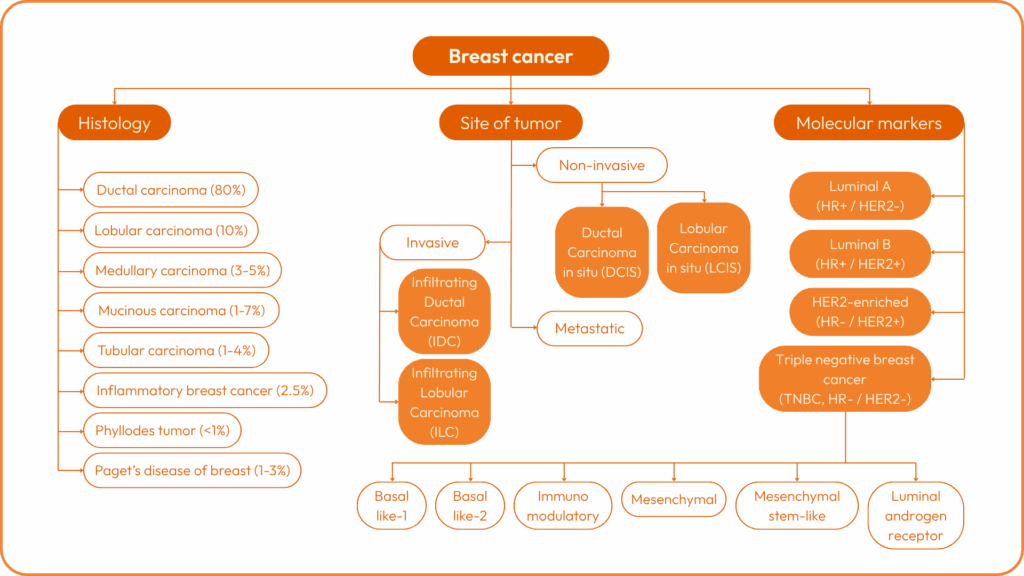
There are several types of breast cancer:
Each cancer is then characterised by a stage from I to IV, and by a grade from I to III. Stage 4 represents metastatic cancer.
Cancer subtypes can then be determined on the basis of the expression (or otherwise) of certain molecular biomarkers:


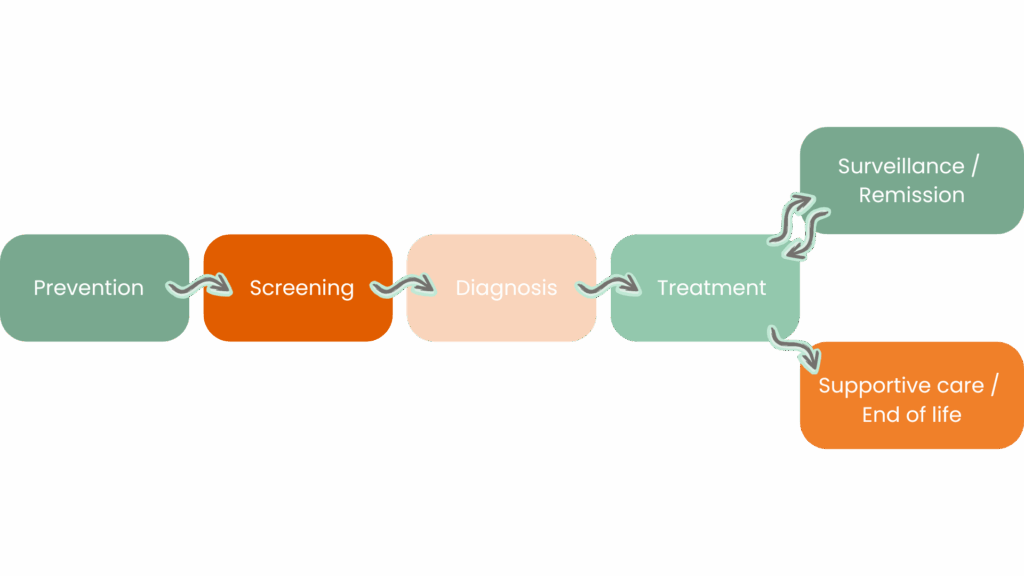
The screening phase is mainly carried out by mammography in primary care.
During the diagnostic phase, several consultations are carried out:
The diagnosis is announced on the day or within a week if the biopsy had to be taken during the initial consultation.
Once the biopsy has been taken, the work of the anatomopathology laboratory begins:
For example, HES (Haematoxylin – Eosin – Saffron) staining is used for standard diagnosis, whereas IHC (Immunohistochemistry) staining is used to identify target proteins or antigens and therefore cancer subtypes.
In our challenge, we work on IHC slides.
The staining is then carried out using a combination of machine and antibody. The choice of antibody depends on the target.
In the case of HER2, we need to :
Morgane MENARD
Head of the IMPULSE by ICO Accelerator
morgane.menard@ico.unicancer.fr
Antoine GUYONVARCH
Innovation and digital health project manager
antoine.guyonvarch@ico.unicancer.fr
